Knowing a patient achieved an above average medication adherence rate can be a promising sign.
It can also be a deceptive one.
In the adjacent graphic, adherence rates exceeding 90% were recorded for 4 patients in a clinical trial.1 This single data point would be available from traditional pill counts, PK draws, and other methods.
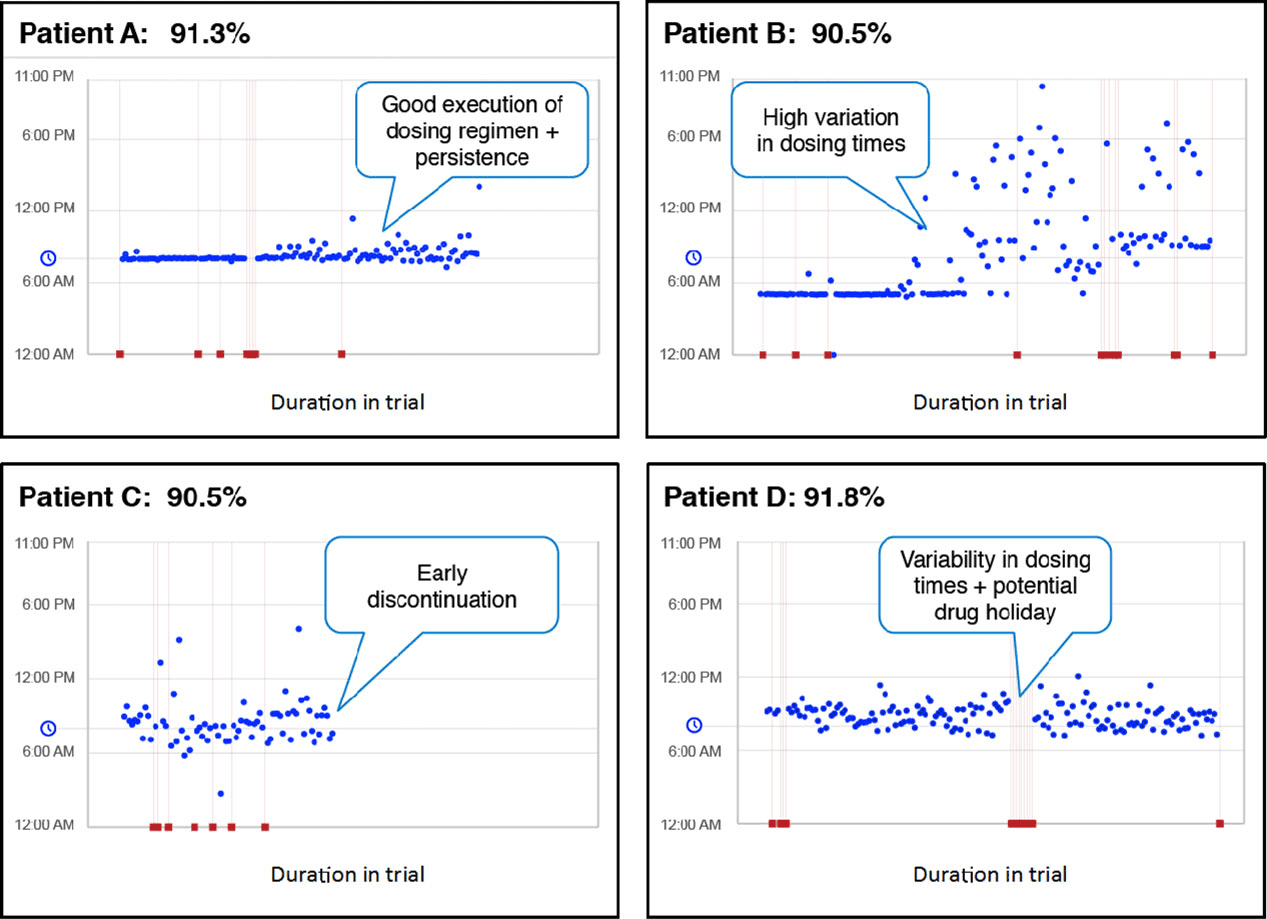
In this case, it was calculated using AiCure’s smartphone-based adherence application. Despite the impressive adherence rates, AiCure data revealed only Patient A was fully “on protocol.”
The other patients posed risks to study integrity with dosing behaviors including early discontinuation, variability in dosing times, and a potential drug holiday. Patient B’s variability might result in having too little dose to be meaningful or too great a dose, creating a level of toxicity which might have been mitigated through an alert to the site (and ultimately the participant) about the importance of being consistent on the dose time. Patient C’s early discontinuation might have been alleviated had the site intervened earlier on the warning signs of missed dose. And Patient D’s drug holiday also might have created a warning and intervention from the site to limit to a one day miss, minimizing the risk of an insufficient amount of drug. These details are a few of the deeper insights made possible by using AiCure adherence technology.
All of these patients appear to have similar adherence levels. But will they all have the same outcome? And what does this do for study analysis?
AiCure evaluates a broad spectrum of patient dosing behaviors to drive more accurate and more powerful decisions, enabling interventions that are specific to each patient at risk of non-adherence.
AiCure Patient Connect is a powerful mobile app for capturing and analyzing dosing behavior using smartphone video, computer vision and machine learning.
Learn more by downloading this fact sheet.
1 Shiovitz, Mitigating the Effects of Nonadherence in Clinical Trials, J Clin Pharmacol, 2016
